You can contact LEARNZ, part of CORE Education, at:
Postal Address:
PO Box 13 678,
Christchurch 8141,
New Zealand
Water can exist as a solid, liquid and gas here on Earth. Changes in the state of water mean that water molecules can move between the air, ground, plants, lakes, rivers and oceans.
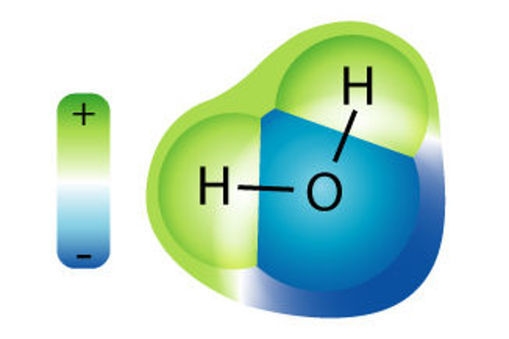
A molecule is a group of two or more atoms that bond or ‘stick’ together. Water is a molecule made up of two hydrogen (H) atoms and one oxygen (O) atom. These atoms are chemically bonded together. The H and O are symbols for the atoms that make up water. People often call water H2O.
Water can change from a liquid to a solid or a gas and back to a liquid, over and over again.
Not all substances can do this as heat can chemically change some molecules, for example if you burn wood the molecules are permanently changed, and this change cannot be reversed. This is known as a chemical change.
Unlike chemical changes, physical changes in state do not permanently change the molecules. If you change the state of water, you don't change the amount of water or the molecules that make up this water.
A water molecule is always H2O whether it is liquid water, ice or water vapour. The only thing that changes is the movement of the water molecules:

Rivers, groundwater, lakes, oceans and rain represent the liquid state of water.
When water is heated it evaporates and becomes water vapour - a gas. We only see water vapour when it cools and condenses into clouds.
When water cools into a solid it is seen as snow, hail or ice.
In its gaseous state, water vapour is evaporated by the Sun’s solar radiation from the surface of water bodies like oceans or lakes, and from the surface of plants and the land. Water vapour can also evaporate directly from its frozen state.
Snow and ice represent the solid form of water. They can be found in the Earth’s polar icecaps and on top of high mountains. Some of the snow and ice melts and turns into liquid water. In the polar regions, ice can stay frozen for thousands of years.
Only a very small amount of the total amount of water (about 0.3%) is drinkable water.
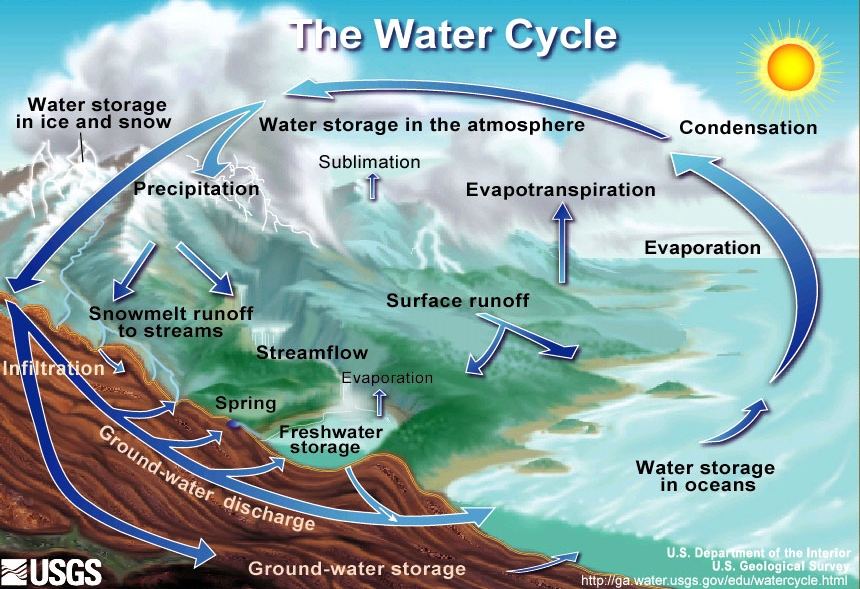

If you leave some water on a saucer by a window, it will over time evaporate. When this water cools it will become visible again as water droplets on the window or window sill. If it gets really cold it may freeze. Changes in temperature change the state of water, creating the water cycle.
Ready for a quiz? Try the Water Cycle interactive activity.
Water evaporates only if there is enough thermal (heat) energy available for the water molecule to vibrate so much that the molecules ‘break’ out of their liquid structure and turn into a gas.
But why do the oceans not dry up? In fact, most evaporation occurs from ocean water, but much of the evaporated water rains back into the oceans again. Some falls on the land surface and might spend some time there as ice, snow, groundwater or in streams, or it may be stored in lakes before it returns back to sea.
This journey is called the hydrological cycle or water cycle. It describes the exchange of water in every form between the Earth’s systems and is part of what makes the Earth so unique. Understanding things that can affect the water cycle helps us to understand how climate change will affect people.
This cycle is complex and small changes in things like temperature can cause large changes in the whole system.